
How Much Protein is Too Much? EatPlantBased
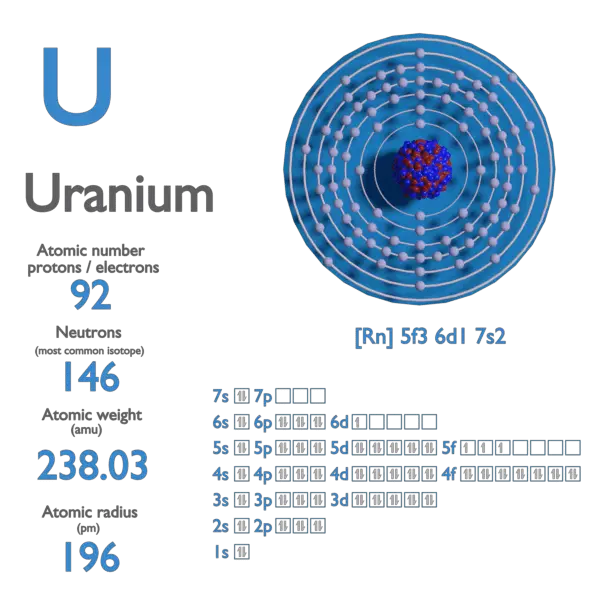
orthorhombic ( oS4) Uranium is a chemical element; it has symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactively decays, usually by emitting an alpha particle.

Uranium and Thorium Australia’s Energy Commodity Resources 2021
The engineered protein is thermally stable and offers very high affinity and selectivity for uranyl with a Kd of 7.4 femtomolar (fM) and >10,000-fold selectivity over other metal ions. We also.

INFOGRAPHIC 9 Fascinating Facts About Uranium Advancing Mining
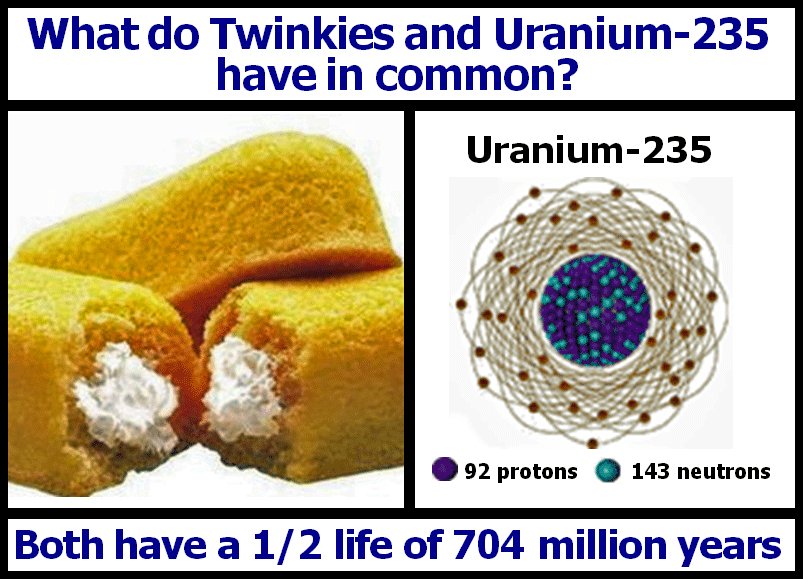
Uranium is a radioactive element primarily used in nuclear reactions. Uranium can release a huge amount of energy when it undergoes fission. One gram of uranium-235, the most commonly used isotope for nuclear power, can release about 8.22 × 1010 joules (~20 billion calories) of energy when it undergoes complete fission.

How The Uranium Market Will Evolve For The PostPandemic Energy World
Uranium is a common element in Earth's crust (soil, rock) and in seawater and groundwater. Uranium has 92 protons in its nucleus. The isotope2 238U has 146 neutrons, for a total atomic weight of approximately 238, making it the highest atomic weight of any naturally occurring element. It is not the most dense of elements, but its density is.

What is uranium enrichment level and why does it matter? YouTube
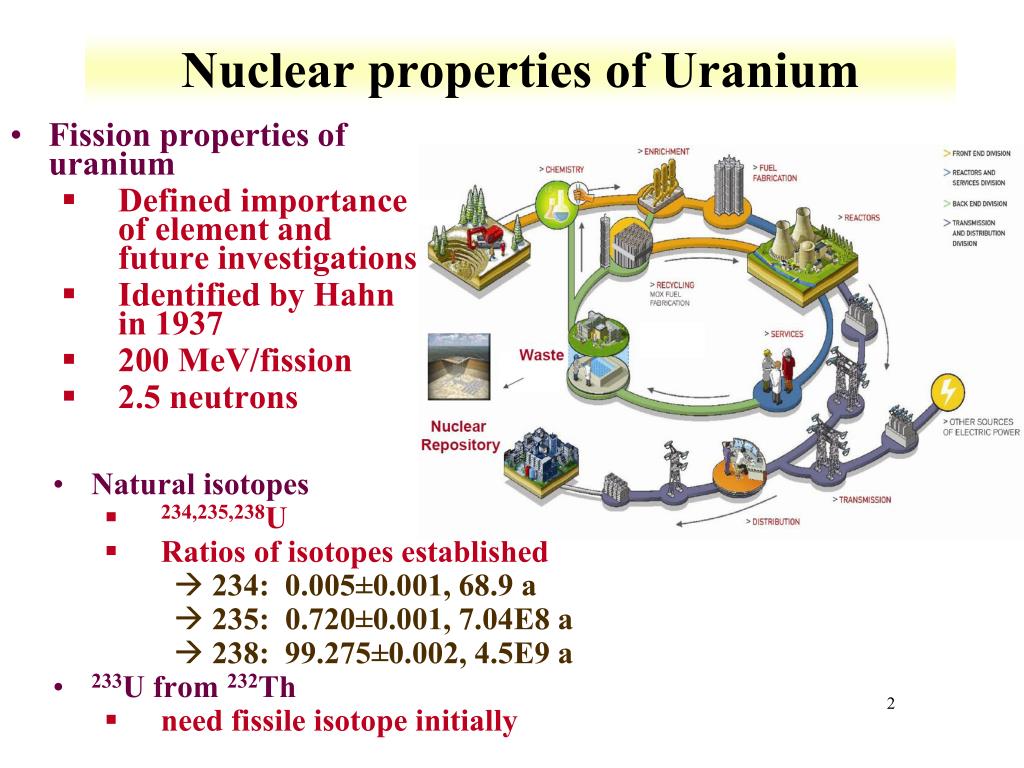
There are three natural isotopes of uranium — uranium-234 (U-234), uranium-235 (U-235) and uranium-238 (U-238). U-238 is the most common one, accounting for around 99 per cent of natural uranium found on earth. Most nuclear reactors use fuels containing U-235, however, natural uranium typically contains only 0.72 per cent of U-235 and, most reactors need a higher concentration of this.

Définition Uranium
Uranium is an alpha-emitting, radioactive, heavy metal that occurs naturally in nearly all rocks and soils. Twenty-two isotopic forms of uranium have been identified, mainly associated with nuclear reactor operations or high-energy physics experiments; the most prevalent isotopes found in the environment are the three naturally-occurring isotopes: 234U, 235U, and 238U. Most uranium isotopes.

What Is Uranium And Its Properties / Uranium Facts, Symbol, Discovery, Properties, Uses The
1. Introduction. Exposure to uranium may lead to health risks due to its chemical and radiological toxicity [].Various epidemiological and laboratory studies have shown that environmental and occupational levels of uranium exposure can lead to a wide range of health problems [2,3,4].Uranium entering the human body is mainly in the form of hexavalent oxide uranyl ions.
Uranium Periodic Table Mass Number Elcho Table
The widespread use of uranium for civilian purposes causes a worldwide concern of its threat to human health due to the long-lived radioactivity of uranium and the high toxicity of uranyl ion (UO 2 2+).Although uranyl-protein/DNA interactions have been known for decades, fewer advances are made in understanding their structural-functional impacts.
PPT Uranium Chemistry and the Fuel Cycle PowerPoint Presentation, free download ID3797683
Uranium is a metallic element with radioactive properties. Pic credit: Pixabay. For 1 kg of uranium-235 to release energy equivalent to approximately 20 billion Calories, all the atoms in it must undergo fission. There are about 2.56 X 10 24 atoms in a kilogram of uranium-235.

World's Top UraniumProducing Countries 1970 2018 YouTube
Uranium enrichment for nuclear energy produces uranium that typically contains 3% 235 U. Uranium enrichment for a number of other purposes, including nuclear weapons, can produce uranium that contains as much as 97.3% 235 U and has a higher specific activity (~50 µCi/g). The residual uranium after the enrichment process is called "depleted.

TOUCH this image Uranium 92 Rudowski 5th period by Nick Rudowski Minerals and gemstones
The world's conventional identified uranium resources amounted to 8 070 400 tonnes of uranium metal (tU) as of 1 January 2019. These represent all reasonably assured and inferred uranium resources that could be recovered at market prices ranging from 40 to 260 USD/KgU (equivalent to 15 to 100 USD/lb U 3 O 8). Compared to the total reported in.
How many calories are in uranium? What you need to know
Here, a systematic survey of the structural features of the uranyl sites observed in protein crystal structures deposited in the Protein Data Bank is reported. Beside the two uranyl oxygens, which occupy the axial positions, uranium tends to be coordinated by five other oxygen atoms, which occupy the equatorial vertices of a pentagonal.

How Much Daily Protein Intake You Should Have? HealthHelp
The widespread use of uranium for civilian purposes causes a worldwide concern of its threat to human health due to the long-lived radioactivity of uranium and the high toxicity of uranyl ion (UO 2 2+).Although uranyl-protein/DNA interactions have been known for decades, fewer advances are made in understanding their structural-functional impacts.

Uranium Production by Country 2023 Wisevoter
Uranium is a very important element because it provides us with nuclear fuel used to generate electricity in nuclear power stations. It is also the major material from which other synthetic transuranium elements are made. Naturally occurring uranium consists of 99% uranium-238 and 1% uranium-235.
Is Uranium 235 Used For
Nuclear Fuel Facts: Uranium. Uranium is a silvery-white metallic chemical element in the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium has the highest atomic weight of all naturally occurring elements.

How many calories 1g uranium? YouTube
This protein was more abundant in the uranium-tolerant strains under uranium stress (FC values: 1.7 and 4.7 in strain ViU2A; 2.0 and 3.1 in strain HG3; and 1.5 and 5.3 at 4 and 24 h in strain A9.
- Temperature In Netherlands In December
- Wat Doet Een Functioneel Applicatiebeheerder
- Buggy Clips For Changing Bag
- Films Et Séries Tv Avec Audrey Fleurot
- Monica Geuze Televizier Ring 2023
- Wat Is De Hoofdstad Van Dubai
- Wat Is Belangrijk Bij Samenwerken
- Actors Of The Addams Family
- Psv Eindhoven Vs Ajax Amsterdam Matches
- Hoe Oud Is Milo Ter Reegen