
Prospective Process Validation
This guidance outlines the general principles and approaches that FDA considers appropriate elements of process validation for the manufacture of human and animal drug and biological products.
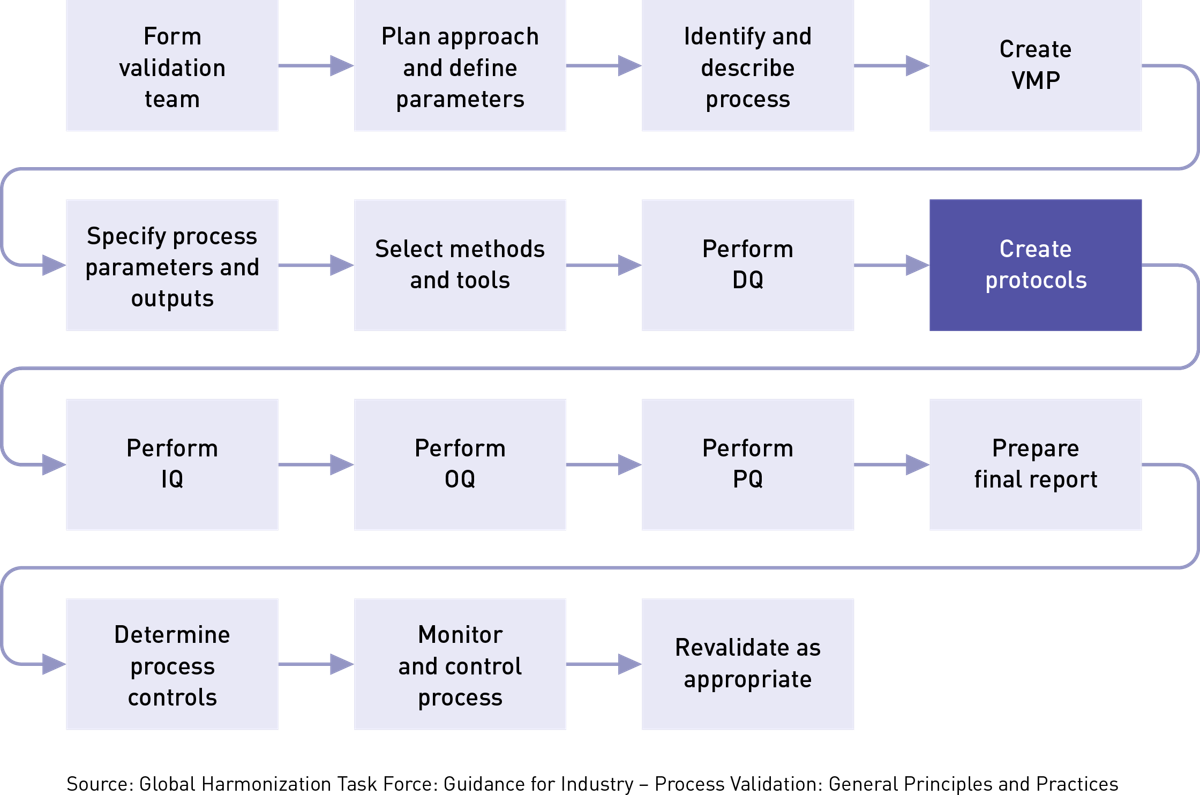
Master Validation Plan Template Medical Device
The lifecycle approach to process validation is a significant paradigm change compared to the 1987 approach. The 1987 approach emphasized documentation of the demonstrated (three lots) process; the 2011 approach emphasized Stage 1 understanding and Stage 3 monitoring. Stage 2 was realized to be a "snapshot in time" providing little.

PPT Overview of Validation Requirements in Pharmaceutical Industry Kaushik Desai Chairman
ISPE's guide focuses on the lifecycle approach to process validation which determines the product's quality at every stage and overall reproducibility. "Process validation is an essential part of the pharma industry because back in the early 2000s there was a drug shortage that was caused by companies not understanding what it took to get.
Process Validation The Essential Guide to Ensuring Product Quality and Compliance Pharma GxP
Process validation in the pharmaceutical industry is a systematic approach to confirm that a process consistently produces a product meeting its predetermined specifications and quality attributes. GxP process validation encompasses various regulations and guidelines that ensure the quality, safety, and efficacy of products in regulated industries.

Cleaning validation of production equipment Visual inspection, accreditation of staff in
In today's pharmaceutical industry, process validation relies on information and knowledge from product development activities to ensure patient requirements are translated into product attributes. A key to success is establishing a comprehensive science-based process design that focuses on understanding sources of variability. Translating.

Process validation Pharma Solutions Ltd
This Guidance provides useful support for the implementation of a lifecycle approach to pharmaceutical process validation (PV). It contains information that enables manufacturers to implement globally-. Terminology definitions that are widely recognized by the industry should be considered when establishing internal definitions. Hence, the.
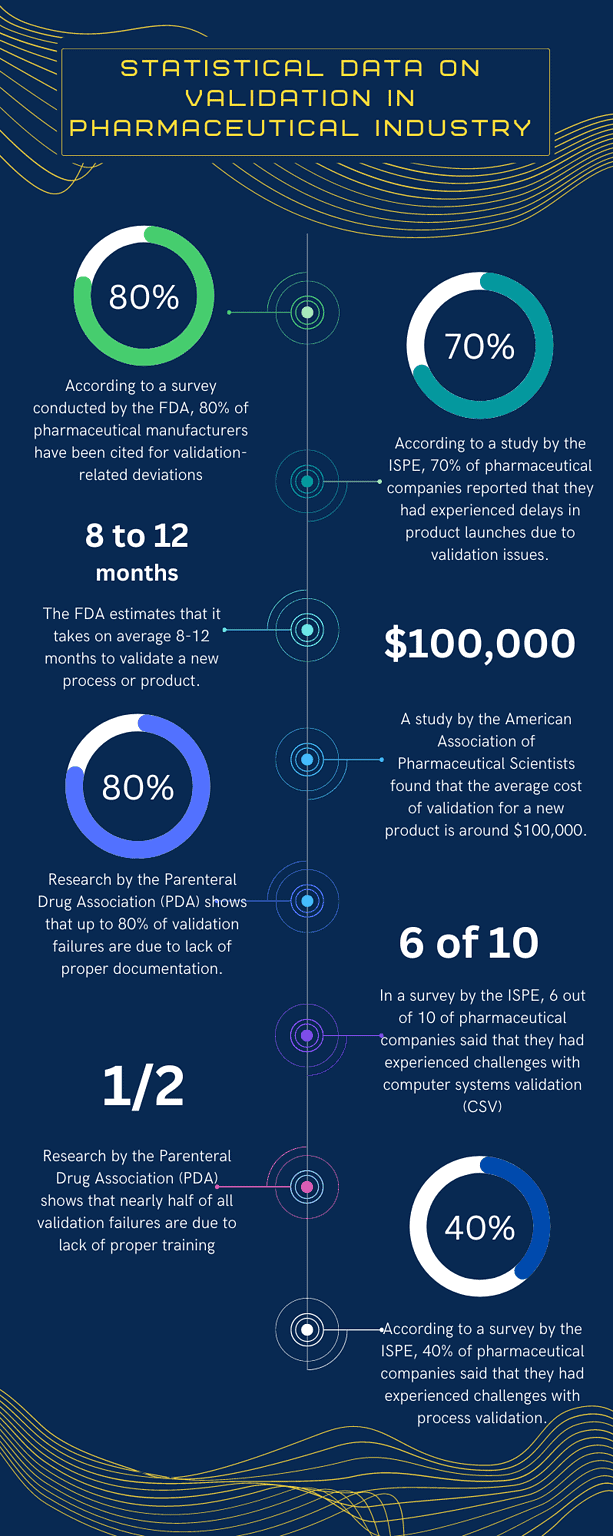
Concept of process Validation in Pharmaceutical Industry overview
PROCESS VALIDATION IN PHARMACEUTICAL INDUSTRY; AN OVERVIEW. S. Sharma, Gurpreet Singh. Published 14 July 2013. Medicine. Journal of Drug Delivery and Therapeutics. Quality cannot be adequately assured by in-process and finished inspections and testing but it should be built in to the manufacturing process.

Process Validation in Pharmaceutical Manufacturing Validation in Pharmaceuticals YouTube
In Pharmaceutical industry, Product quality is of higher significance. To ensure that product meets its predetermined quality attributes, identification and control over critical parameters during manufacturing operations is of utter importance.. Process validation is continuous process which establishes evidence that quality and compliance.

Process Validation ISPE International Society for Pharmaceutical Engineering
Traditional process validation is normally performed when the pharmaceutical development and/or process development is concluded, after scale-up to production scale and pri or to marketing of the finished product. As part of the process validation lifecycle , some process validation studies may be

Cleaning Validation Regulatory Guidelines for the Pharmaceutical Industry YouTube
1. Purpose. This document is for anyone involved in the fabrication, packaging/labelling, testing, importation, distribution and wholesaling of drugs. It describes how to properly qualify and validate drug manufacturing processes, facilities, equipment, utilities and analytical methods. The application of this document will vary depending on.

Process Validation and Sterility Assurance Relations and Requirements American Pharmaceutical
In the life-science industry, process validation is a regulatory requirement and technique to ensure a consistent and high quality product.. Regulations for Finished Pharmaceuticals, 21 CFR Parts 210 and 211. Good Manufacturing Practice (GMP) Regulations for Medical Devices, 21 CFR Part 820, Quality System Regulation..

PPT Producing a Pharmaceutical or Biopharmaceutical PowerPoint Presentation ID419859
FDA regulations require that process validation procedures be established and followed (§ 211.100) before a batch can be distributed (§§ 211.22 and 211.165). routine production. It should also.
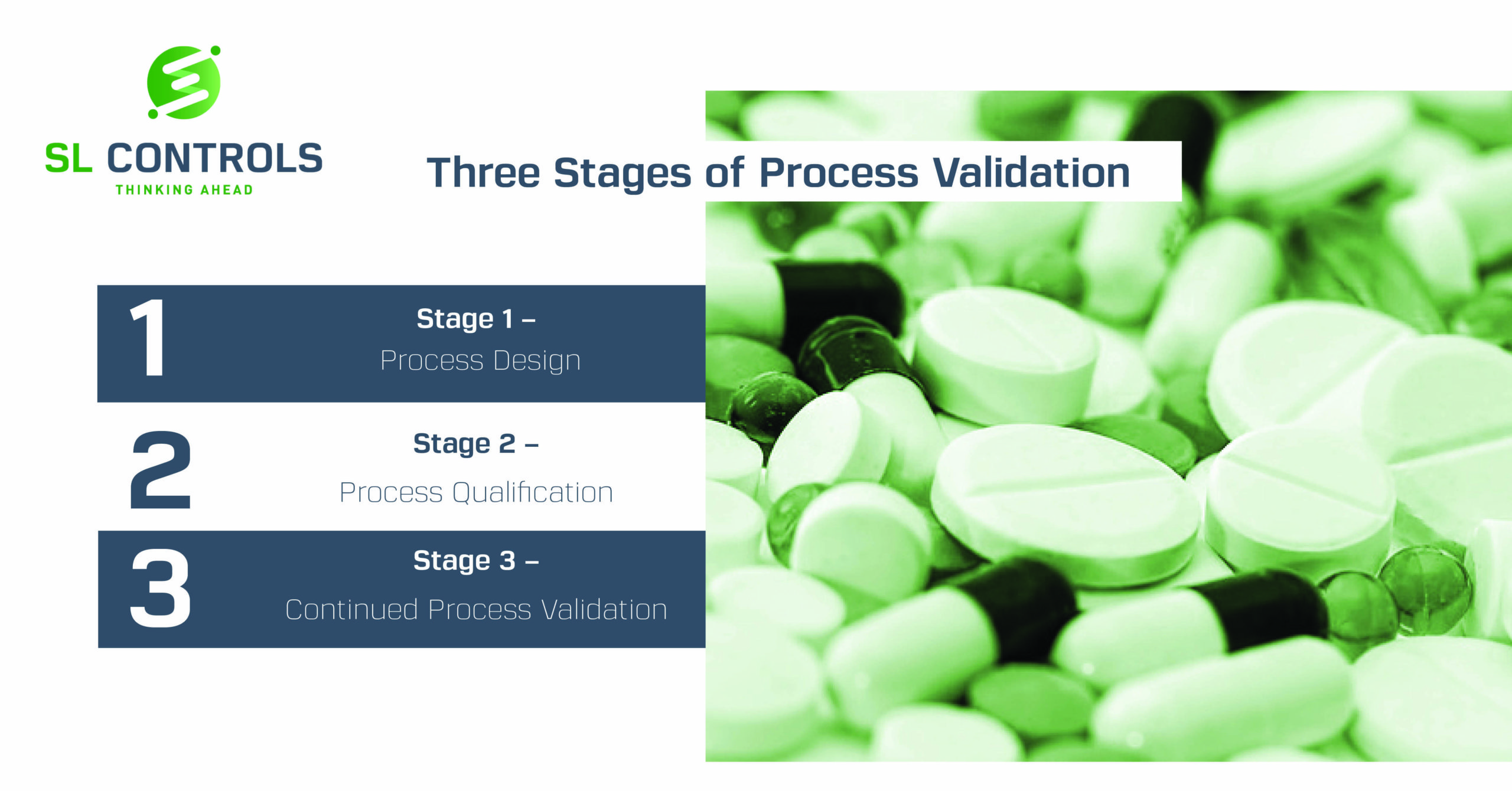
The 3 Stages of Process Validation Explained SL Controls
Process validation involves a series of activities taking place over the lifecycle of the product and process. Thisguidance describes process validation activities in three stages. Stage 1 - Process Design: The commercial manufacturing process is defined during this stage based on knowledge gained through development and scale-up activities.

PPT Overview of Validation Requirements in Pharmaceutical Industry Kaushik Desai Chairman
PHARMACEUTICAL PROCESS VALIDATION: AN OVERVIEW. MAHAR P, VERMA A. Department of pharmacy, GRD (PG) IMT, Dehradun-248001, Uttarakhand. Accepted Date: 30/0 6/2014; Published Date: 27/08/2014.

Pharmaceutical Validation Guide How to Get It Right Dickson
In the field of pharmaceutical manufacturing, process validation aims for excellence in product quality, safety, and efficacy. It is a systematic approach that goes beyond mere compliance, encompassing a series of stages to ensure that each step of the manufacturing process consistently produces a product that meets predefined specifications.

Process validation of pharmaceuticals Verification And Validation Tablet (Pharmacy)
The current chapter is based on guidance for industry entitled Process Validation: General Principles and Practices published by the US Food and. (FDA)'s (2011) guidance on Process Validation provides a stepwise framework on pharmaceutical process validation that aids in understanding the critical factors concerning initial input, process.